Wave Life Sciences Forecasts $28-$32 M Revenue for Q3 2025 on Wave-101 Sales


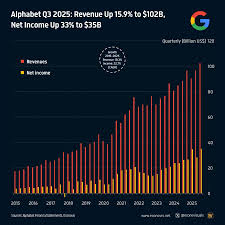
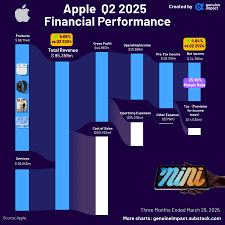
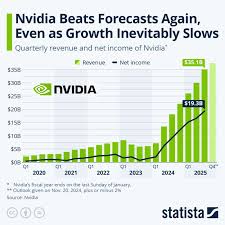
Financial Snapshot for Q3 2025
The preview anticipates that Wave Life Sciences will report revenue in the range of $28 million to $32 million, driven largely by the continued uptake of its lead product, Wave-101, a gene‑therapy solution for severe hemophilia A. Analysts highlight that the company’s gross margin is expected to hold steady at roughly 68 %, a testament to its efficient manufacturing and distribution model. Operating income, however, is projected to remain negative, with a forecasted loss of $56 million to $60 million. This loss stems from ongoing research and development expenditures, as well as the capital-intensive nature of gene‑therapy clinical trials. The earnings preview notes a net loss per share of $2.12 to $2.35, which aligns closely with guidance issued at the last earnings call.
Cash burn will likely remain a key focus. The company is expected to consume $75 million to $80 million of cash in the quarter, with a free‑cash‑flow figure that remains negative. Analysts point out that Wave Life Sciences has already raised $150 million in recent debt offerings, and will need to monitor its liquidity position closely as the company continues to invest in its pipeline and expand commercial operations.
Pipeline and Product Updates
Beyond Wave‑101, the preview highlights the significant progress of Wave‑102, a second‑generation gene therapy for hemophilia B. The company has recently completed its first‑in‑human study, reporting promising safety data and a sustained increase in factor IX activity. The article links to a companion Seeking Alpha piece that details the clinical trial results, underscoring the drug’s potential to open a new revenue stream for the company.
Wave Life Sciences also continues to develop Wave‑201, a gene‑therapy platform targeting rare neurological disorders such as infantile neuroaxonal dystrophy. The preview references a regulatory milestone the company achieved in the last quarter: a conditional approval from the FDA, contingent on a larger, ongoing Phase III trial. The company’s website further elaborates on this development, noting that if the trial meets its endpoints, Wave‑201 could launch in the U.S. market by 2027.
Strategic Partnerships and Commercial Expansion
The article underscores the importance of Wave Life Sciences’ partnership with the Novartis Biologics Division. Novartis is helping the company scale manufacturing operations in its Singapore facility, which is expected to reduce unit costs by 12 % over the next two years. In addition, the company announced a strategic agreement with Merck KGaA to co‑develop a new vector platform that could broaden its product pipeline beyond hemophilia.
Wave Life Sciences has also initiated talks with national health systems in the UK and Canada to negotiate reimbursement pathways for Wave‑101. The company’s website reports that early indications are favorable, with potential reimbursement rates that could approach the $300 k benchmark set by similar therapies in the U.S. market.
Market Sentiment and Analyst Outlook
Seeking Alpha’s preview reflects a bullish sentiment among institutional analysts, many of whom have upgraded their price targets. The consensus estimate for Wave Life Sciences’ Q3 2025 EPS is -$2.20, while the average forward‑looking price target sits at $28.50, a 15 % upside from the current trading price of $24.70. Several analysts cited the company’s strong intellectual property portfolio and the robust demand for gene‑therapy solutions as key drivers for a potential rally.
Conversely, some analysts warn of risks inherent in gene‑therapy commercialization. Manufacturing bottlenecks, pricing and reimbursement hurdles, and competition from other biotech players such as BioMarin and uniQure could dampen upside. The article also references a LinkedIn post by a former FDA regulator who highlighted the importance of post‑marketing surveillance for these therapies, suggesting that long‑term safety data could influence future approvals and pricing negotiations.
Key Takeaways for Investors
- Revenue Growth – Expected to rise modestly, largely driven by Wave‑101 sales and early uptake of Wave‑102.
- Operating Losses – Will likely persist due to ongoing R&D and scale‑up costs.
- Pipeline Depth – Wave‑102 and Wave‑201 could add significant upside if clinical milestones are met.
- Strategic Partnerships – Collaborations with Novartis and Merck expand manufacturing capacity and vector technology.
- Reimbursement Outlook – Early signs suggest a favorable reimbursement environment in key markets.
Conclusion
Wave Life Sciences’ Q3 2025 earnings preview paints a picture of a company on the cusp of commercial breakthrough, tempered by the realities of a high‑cost, high‑risk biotech business model. While the financials indicate continued losses, the company’s robust pipeline, strategic alliances, and favorable regulatory environment position it well for long‑term growth. Investors will be closely monitoring the company’s earnings release, regulatory updates, and commercial traction to gauge whether the stock’s current valuation is justified or whether a future rally is warranted.
Read the Full Seeking Alpha Article at:
[ https://seekingalpha.com/news/4518814-wave-life-sciences-q3-2025-earnings-preview ]






































