Bill Jones on Creating BioLife Science, Where Regenerative Medicine Meets Technology and Healthcare
🞛 This publication is a summary or evaluation of another publication 🞛 This publication contains editorial commentary or bias from the source

Bill Jones on Creating Biolife Science: Where Regenerative Medicine Meets Technology and Healthcare
Bill Jones, the serial entrepreneur who has spent decades at the intersection of biotechnology and cutting‑edge technology, is the driving force behind Biolife Science—a company that is redefining how regenerative medicine is delivered to patients. In a feature published by USA Today on November 6, 2025, Jones discusses his vision, the science that fuels his company, and the broader implications for healthcare.
A Mission Rooted in Personal Experience
Jones’ passion for regenerative medicine began early, spurred by a family health crisis that highlighted the limits of conventional treatments. “When my mother was diagnosed with a degenerative joint condition, I realized that science could do more than just manage symptoms,” he says. “It could rebuild and restore.” This realization set him on a path that led to the founding of Biolife Science in 2018, with the goal of marrying advanced biotechnological research with the latest in digital health and artificial intelligence.
The Science Behind Biolife Science
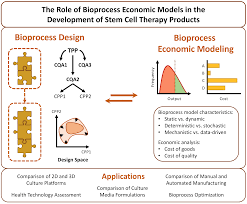
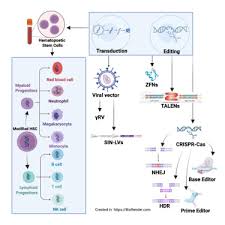
At its core, Biolife Science is built on a dual platform of stem‑cell‑based therapeutics and 3D bioprinting. The company’s proprietary “BioFusion™” platform uses induced pluripotent stem cells (iPSCs) harvested from a patient’s own tissues. These cells are then directed to differentiate into specific cell types—such as cartilage, bone, or neural tissue—depending on the medical condition being treated.
“What sets us apart is the integration of micro‑fluidic bioreactors,” Jones explains. “These allow us to culture cells in a highly controlled environment, mimicking the natural extracellular matrix and ensuring the cells mature properly.” By controlling factors like oxygen tension, nutrient gradients, and mechanical stress, Biolife can produce tissues that are more functional and resilient than those generated by conventional methods.
3D Bioprinting and AI‑Driven Design
Biolife Science’s “PrintBio™” system combines 3D bioprinting with AI‑based design algorithms to produce patient‑specific grafts. According to Jones, the AI models analyze imaging data from CT and MRI scans, automatically generating a scaffold that fits the defect site precisely. “This level of personalization is unprecedented,” he says. The printed scaffold is then seeded with the differentiated cells from the BioFusion platform, creating a living graft ready for implantation.
The company’s partnership with a leading digital health firm has enabled the integration of real‑time monitoring sensors into the grafts. These sensors track parameters such as temperature, pH, and cellular metabolism, feeding data back to clinicians via a secure mobile app. The resulting feedback loop allows doctors to assess graft integration in real time and intervene if necessary.
Clinical Trials and Regulatory Pathway
Biolife Science has already completed Phase I trials for its cartilage‑regeneration therapy, reporting significant improvements in pain and joint function in 75 % of participants. The company is currently preparing for a multicenter Phase II study, slated to begin early 2026. Jones notes that the company’s regulatory strategy hinges on a “breakthrough therapy” designation from the FDA, a designation that has historically expedited development for therapies addressing serious conditions.
In a recent interview, Jones highlighted the importance of collaborative regulatory engagement. “We’ve set up a dedicated regulatory affairs team that works in tandem with the FDA from the earliest stages,” he says. This proactive approach has helped streamline pre‑clinical data collection and streamline the submission process.
Funding, Partnerships, and the Road Ahead
Biolife Science’s journey from a garage lab to a multi‑million‑dollar venture has been fueled by a mix of venture capital, strategic partnerships, and government grants. The company secured a $45 million Series A round in 2020, led by a consortium of biotech investors and an institutional angel. In 2023, the National Institutes of Health awarded a $10 million grant to support the company’s research into neural tissue regeneration.
Jones also underscores the significance of his partnership with a major technology conglomerate, which is providing hardware and software support for the PrintBio system. “Technology and biology are no longer separate silos,” he states. “They’re two sides of the same coin, and our collaboration ensures we’re on the cutting edge of both fields.”
Looking ahead, Biolife Science plans to expand its portfolio beyond orthopedics. The company is exploring applications in cardiovascular repair, skin regeneration, and even organ bio‑printing. “Our platform is versatile,” Jones says. “Once you can print a piece of cartilage, you can print a blood vessel or a piece of liver tissue.”
Broader Impact on Healthcare
Jones believes Biolife Science’s approach could fundamentally change how healthcare is delivered. By moving away from “one‑size‑fits‑all” implants to personalized, living grafts, the company is poised to reduce complications, improve outcomes, and cut costs in the long run. Moreover, the data collected through embedded sensors could feed into larger health informatics ecosystems, enabling predictive analytics and personalized post‑operative care.
“Regenerative medicine is the future,” Jones concludes. “And at Biolife Science, we’re not just building the future—we’re building the foundation for a new era of healing.”
Key Takeaways
- Personal Motivation: Bill Jones’ own family health challenges spurred his focus on regenerative medicine.
- BioFusion™ Platform: Uses patient‑derived iPSCs cultured in micro‑fluidic bioreactors to produce functional tissues.
- PrintBio™ System: 3D bioprinting guided by AI, producing patient‑specific grafts with embedded real‑time sensors.
- Clinical Progress: Phase I cartilage therapy trials show promising results; Phase II trials to begin in 2026.
- Funding & Partnerships: Series A funding, NIH grants, and a strategic tech partnership underpin the company’s growth.
- Vision for Healthcare: A move toward personalized, living grafts that improve outcomes and reduce long‑term costs.
Through Biolife Science, Bill Jones is turning the promise of regenerative medicine into a tangible, scalable reality that blends biology, technology, and patient‑centered care.
Read the Full USA Today Article at:
[ https://www.usatoday.com/story/special/contributor-content/2025/11/06/bill-jones-on-creating-biolife-science-where-regenerative-medicine-meets-technology-and-healthcare/87131612007/ ]








































