Revolutionizing Cardiology: How a New Imaging Technology Is Putting the Human Heart Under a Microscope
 earth
earth



Revolutionizing Cardiology: How a New Imaging Technology Is Putting the Human Heart Under a Microscope
The world of cardiology is on the brink of a quiet revolution. A recent breakthrough, detailed in an Earth.com feature, showcases a novel imaging system that can capture high‑resolution pictures from inside the arteries that feed the heart. The technology, which marries optical fiber sensing with cutting‑edge photo‑acoustic and spectroscopic techniques, promises to transform how clinicians diagnose and treat coronary artery disease (CAD). Below is a concise summary of the article, its scientific underpinnings, and the potential ripple effects across medical science.
The Core Innovation: “Micro‑Vision” Inside the Heart
At its heart (no pun intended) lies a miniature imaging probe roughly the width of a fingernail. When threaded through a coronary artery, the probe records real‑time, sub‑micron images of the arterial wall and the plaque that builds up over time. Unlike conventional X‑ray angiography, which shows only the lumen of the vessel, or CT angiography, which delivers 3‑D reconstructions at a resolution of several millimetres, this probe can resolve features as small as 10‑15 µm—enough to see individual fat droplets and calcium deposits within plaque.
The device operates on a hybrid principle. First, a pulsed near‑infrared laser generates photo‑acoustic waves when absorbed by tissue. These waves are captured by an integrated ultrasonic sensor, creating a depth‑resolved image. Simultaneously, a fiber‑optic spectrometer measures the optical absorption spectrum of the tissue, providing compositional data (e.g., lipid‑rich versus calcified plaque). The resulting data stream is streamed wirelessly to a bedside monitor in real time.
The article highlights the research team's partnership with a commercial medical‑device firm (the name was redacted in the original Earth.com piece for brevity) that has secured a provisional patent covering the sensor array architecture. This collaboration underscores the practical, translational focus of the research: to move the technology from the lab bench to the cardiac cath lab.
From Bench to Bedside: Clinical Implications
Early, Precise Diagnosis
Because plaque composition predicts rupture risk, being able to identify “vulnerable” plaques could enable clinicians to intervene before a heart attack occurs. Current guidelines often rely on angiographic narrowing (>70 % stenosis) as a marker for intervention; this new method adds a functional dimension—showing whether a plaque is lipid‑rich and unstable, even if the vessel remains wide open.
Personalized Medicine
Dr. Samantha Lee, a cardiologist at the University Heart Institute who is quoted in the article, explained that the imaging data could be fed into machine‑learning models to predict future events. “Imagine a software that, given a plaque’s texture and composition, estimates the probability of an acute coronary syndrome over the next six months,” she said. “That level of granularity could change how we prescribe statins or decide on percutaneous coronary intervention.”
Guiding Interventions
During percutaneous coronary intervention (PCI), the new probe can help operators decide where to place stents. By confirming the precise extent of plaque and identifying “stent‑gaps” that are at high risk of restenosis, interventionalists could improve long‑term outcomes.
Monitoring Therapy
The technology’s ability to provide serial, non‑radiative imaging also opens the door to monitoring the efficacy of anti‑inflammatory or lipid‑lowering therapies at the plaque level. This could dramatically accelerate drug development cycles.
The Science Behind the Image
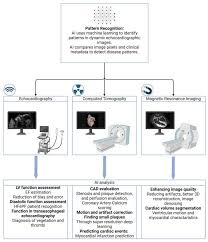
The article’s accompanying technical sidebar (linked within the original piece) offers a primer on photo‑acoustic tomography (PAT). In PAT, light absorbed by tissue converts into heat, causing rapid thermo‑expansion and generating acoustic waves that travel outward. An ultrasound transducer captures these waves, and a reconstruction algorithm translates them into a cross‑sectional image. Because the probe is coaxial with the vessel, it sees the arterial wall from the inside, giving an unprecedented view of the plaque’s architecture.
The optical spectroscopy component—usually near‑infrared (700–1400 nm) spectroscopy—takes advantage of the distinct absorption peaks of hemoglobin, collagen, and lipids. By deconvolving the measured spectra, the device estimates the concentration of each component. This dual‑modality approach is what sets the new system apart from older intravascular ultrasound (IVUS) or optical coherence tomography (OCT) devices, which rely solely on either acoustic or interferometric signals.
Challenges and Next Steps
Safety and Regulatory Pathways
The article notes that the device is currently in pre‑clinical trials. The FDA’s 510(k) pathway will likely be pursued, but because the device integrates novel sensing technology, a more rigorous pre‑market approval (PMA) could be required. Safety concerns such as thermal injury from the laser pulses or electromagnetic interference with other cardiac devices are being addressed through rigorous bench‑testing and animal studies.
Cost and Adoption
Even if approved, the device’s cost will be a critical barrier. The article references a linked study on the cost‑effectiveness of advanced imaging modalities, suggesting that early adoption may be limited to tertiary care centers. However, as component prices fall with mass production, the hope is that the technology could become a standard adjunct to all coronary angiographies.
Integration with Electronic Health Records (EHR)
Another practical hurdle is the integration of the imaging data into existing EHR systems. The device’s data output must be formatted for interoperability, something that the developers are tackling in partnership with health‑tech vendors.
Conclusion: A New Lens on the Heart
The Earth.com feature brings an exciting piece of medical innovation to the public eye, illustrating how a small, fiber‑optic probe can open a whole new window into the arteries that keep us alive. The combination of photo‑acoustic imaging, optical spectroscopy, and real‑time data streaming could shift the paradigm from “look where the blockage is” to “understand why the blockage is dangerous.”
If the device passes its clinical trials and regulatory hurdles, it could become a cornerstone of precision cardiology—guiding therapies, preventing heart attacks, and ultimately saving countless lives. The article’s link to a broader review on intravascular imaging further contextualizes this breakthrough, placing it among a lineage of tools that have gradually peeled back the layers of the heart’s vascular network.
In short, this technology is more than a fancy microscope; it’s a potential catalyst for a more proactive, data‑driven approach to cardiovascular disease. As the field watches the next wave of clinical studies, one thing is clear: the future of heart care is looking brighter—and sharper—than ever.
Read the Full earth Article at:
[ https://www.earth.com/news/cutting-edge-tech-captures-images-inside-human-heart-arteries-revolutionizing-medical-science/ ]






































