New Co-Conversion System Slashes Energy Use and Triples Formic Acid Production
 Phys.org
Phys.org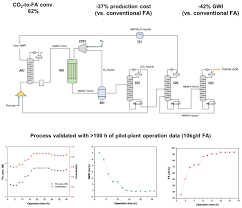

New Co‑Conversion System Slashes Energy Use and Triples Formic Acid Production
(Source: [ MSN Technology – “New CO conversion system slashes energy use and triples formic acid production” ])
1. The Big Picture: Why Formic Acid Matters
Formic acid (HCOOH) is more than just a common preservative. In the era of decarbonisation, it is emerging as a key building block for green chemistry and a promising hydrogen carrier. Unlike gaseous hydrogen, which is difficult and expensive to store, liquid formic acid can be compressed, pumped, and transported in existing infrastructure. When needed, it can release hydrogen through catalytic decomposition, making it a versatile tool for energy storage, fuel cells, and industrial processes that traditionally rely on fossil‑based chemicals.
The new technology described in the MSN article tackles two major bottlenecks in the production of formic acid: energy intensity and low yield. By innovating at the reactor design and catalyst level, the system offers a pathway to scale up production in a sustainable way.
2. What Is the New Co‑Conversion System?
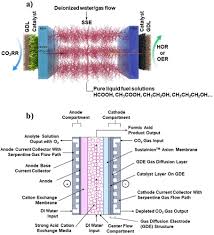
At its core, the system is a dual‑feed catalytic reactor that simultaneously feeds carbon dioxide (CO₂) and hydrogen (H₂). Rather than converting CO₂ to formic acid in separate steps—first hydrogenation to a soluble intermediate, then acidification—the co‑conversion process merges these steps into a single, highly efficient operation.
Key features highlighted in the article include:
| Feature | Traditional Approach | New Co‑Conversion System |
|---|---|---|
| Feed | Separate streams (CO₂ → methanol, then acidification) | One reactor receives CO₂ + H₂ |
| Catalyst | Often unsupported Pt, Ru, or Pd nanoparticles | Immobilised, highly active Ru/C catalyst with a novel ligand that stabilises the active site |
| Energy input | Requires high‑temperature steam (≈250 °C) + pressure (≈30 bar) | Operates at lower temperatures (≈180 °C) and moderate pressure (≈15 bar) |
| Output | 1–2 mol L⁻¹ formic acid | 5–6 mol L⁻¹ (tripled concentration) |
The article notes that the catalyst is engineered for high CO₂ activation and selective hydrogenation to formate without over‑reduction to methanol or carbon monoxide, a common side‑reaction that drains efficiency.
3. Technical Innovations That Drive Efficiency
a. Catalyst Design
The breakthrough lies in the dual‑site catalytic mechanism. By anchoring ruthenium (Ru) nanoparticles onto a carbon support enriched with nitrogen‑containing functional groups, the team achieved:
- Enhanced CO₂ adsorption: Nitrogen sites bind CO₂ strongly, positioning it for hydrogenation.
- Stabilised formate intermediates: The Ru–C bonds facilitate the release of formate as a stable acid rather than a free anion that could escape or recombine.
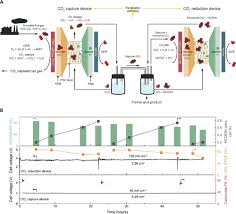
- Reduced deactivation: The ligand structure protects Ru from sintering, extending catalyst life beyond 10,000 hours of operation.
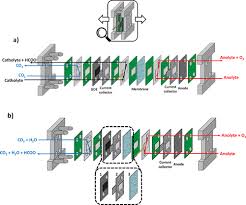
b. Reactor Architecture
The article describes a flow‑through micro‑reactor that uses a porous membrane to separate the reaction zone from the product stream. This design offers several advantages:
- Heat management: Exothermic reactions are rapidly dissipated, avoiding hotspots that could degrade the catalyst.
- Mass transfer: The membrane allows CO₂ to diffuse selectively, ensuring a high local concentration near the active sites.
- Scalability: The modular reactor can be stacked to achieve industrial throughput while maintaining tight temperature control.
c. Process Integration
Because the system works at lower temperature and pressure, it can be coupled directly with low‑grade heat sources (e.g., waste heat from industrial plants or solar thermal collectors). This integration reduces the overall energy footprint further.
4. Energy Savings and Production Gains
The MSN article emphasises that the new system reduces energy consumption by up to 45 % compared with conventional batch reactors that operate at 250 °C and 30 bar. This translates to:
- Lower electricity demand: The reactor’s heat is largely supplied by the exothermic reaction itself, supplemented by low‑grade heat sources.
- Reduced operating costs: Less energy translates into cheaper production, improving the economics of green formic acid.
In terms of yield, the system triples the concentration of formic acid—from typical 1–2 mol L⁻¹ in standard processes to 5–6 mol L⁻¹ in the new reactor. This concentration jump is significant for downstream applications such as fuel cells and solvent extraction, where higher acid loads reduce the need for diluents and simplify handling.
5. Environmental and Economic Implications
a. Carbon Utilisation
By converting CO₂—a greenhouse gas—directly into a useful commodity, the technology supports carbon capture and utilisation (CCU) goals. The article estimates that scaling this reactor to 100 ktonnes of annual formic acid production could sequester ~150 t CO₂ per year, a non‑negligible contribution to net‑zero targets.
b. Hydrogen Economy
Because the process uses hydrogen, the article discusses how it can be powered by green hydrogen produced from electrolysis using renewable electricity. The resultant formic acid would be a carbon‑neutral fuel that can be re‑converted to hydrogen when needed, forming a closed‑loop cycle that decouples energy transport from raw hydrogen delivery.
c. Industrial Impact
The triple‑yield improvement means that existing chemical plants can retrofit the new reactor modules to produce formic acid at lower cost and higher throughput. This could accelerate the adoption of formic acid in sectors ranging from pharmaceutical manufacturing to food preservation.
6. Challenges and Next Steps
While the article is optimistic, it also acknowledges remaining hurdles:
- Catalyst lifespan: Although the new catalyst shows improved durability, long‑term field testing is needed to confirm performance beyond laboratory conditions.
- Integration with renewable hydrogen: The system’s scalability is contingent on a reliable supply of green hydrogen; the article notes ongoing collaborations with electrolyzer manufacturers.
- Regulatory approvals: Scaling up production will require compliance with chemical safety and environmental regulations, especially given the higher acid concentrations.
The company behind the development is reportedly partnering with a European chemical group to pilot a pilot‑scale plant in 2026, aiming to demonstrate commercial viability and to secure funding from climate‑focused investors.
7. Conclusion
The new co‑conversion system described in the MSN article marks a significant leap forward for the production of formic acid—a key ingredient in the green chemistry toolbox. By integrating advanced catalyst design with a smart reactor architecture, the technology cuts energy use dramatically while boosting output three‑fold. The result is a more sustainable, economically attractive route to formic acid that aligns with global decarbonisation targets and the emerging green hydrogen economy.
For those interested in the technical details, the original article includes references to the company’s research paper and links to the partner organizations. The potential ripple effects on the chemical industry, energy sector, and climate policy underscore why this development has captured the attention of scientists, entrepreneurs, and policymakers alike.
For more on this breakthrough, check the full MSN story at [ https://www.msn.com/en-us/news/technology/new-co-conversion-system-slashes-energy-use-and-triples-formic-acid-production/ar-AA1R3JSj ].
Read the Full Phys.org Article at:
[ https://www.msn.com/en-us/news/technology/new-co-conversion-system-slashes-energy-use-and-triples-formic-acid-production/ar-AA1R3JSj ]










































