Quantum Teleportation Explained: Transferring Quantum States
 Popular Mechanics
Popular MechanicsLocales: AUSTRIA, CHINA, SWITZERLAND, UNITED STATES




Understanding Quantum Teleportation and its Limitations
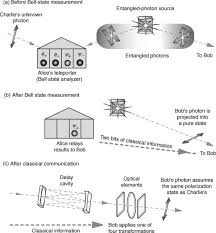
Before diving into repeaters, grasping the basics of quantum teleportation is crucial. The process leverages entanglement, a peculiar quantum phenomenon where two particles become inextricably linked. Measuring a property of one instantly reveals the corresponding property of the other, regardless of the physical separation. Teleportation itself doesn't move the particle; it transfers its quantum state. Imagine particle A holding information you want to transmit. Through entanglement with particles B and C, and a classical communication channel, the state of A can be recreated onto C, effectively teleporting the information.
Yet, this elegant process faces a harsh reality: entanglement is notoriously delicate. The longer the distance between entangled particles, the more prone they are to decoherence, drastically reducing the reliability of the teleported state. This is where quantum repeaters step in as vital intermediaries, acting as relays to extend the reach of quantum communication.
Quantum Repeaters: Extending the Quantum Reach
Quantum repeaters sidestep the limitations of direct entanglement distribution over long distances. Instead of attempting to establish a single, long-range entangled pair, they break the journey into smaller, manageable segments. Multiple entangled pairs are generated across these shorter distances, and then a clever technique called entanglement swapping is employed. Entanglement swapping essentially 'stiches' these shorter entangled links together, effectively creating a longer entangled connection.
This process is often likened to a bucket relay--a single bucket is passed down a line of people. Each person only handles the bucket briefly, but collectively, it travels a considerable distance. Similarly, quantum repeaters handle short entangled links and combine them to create a longer, functional entanglement.
Amir Feizpour, a leading quantum information scientist at Chapman University, succinctly explains it: "It's like relaying a message. Instead of trying to shout across a valley, you have people standing closer together who can relay the message, amplifying it along the way."
The Hurdles in Building Practical Repeaters
Despite the theoretical elegance, building practical quantum repeaters presents formidable engineering challenges. The required precision is staggering. Repeaters need to be incredibly well-isolated to minimize environmental interference and must operate with minimal error rates. Creating and manipulating entangled states with "high fidelity" - meaning with very little error - is paramount. Even minor errors can cascade and corrupt the entire process.
Researchers are exploring several promising avenues. Some are focusing on utilizing specialized crystals to enhance entanglement creation, while others are employing sophisticated error-correcting codes to detect and mitigate errors that inevitably arise. Novel materials science, improvements in cryogenic cooling technology, and advancements in quantum error correction are all proving essential.
Looking Ahead: The Promise of a Quantum Internet
While significant challenges remain, the progress in quantum repeater technology is generating considerable optimism. The development of functional quantum repeaters isn't merely about extending the range of quantum teleportation; it's a critical step toward realizing a truly global quantum internet. Such a network promises unprecedented levels of security for data transmission, as any attempt to eavesdrop would inevitably disturb the quantum state and be immediately detectable. The potential extends beyond secure communication, potentially enabling distributed quantum computing and fundamentally transforming various fields, from finance to medicine.
Although the construction of a fully functional quantum internet remains years away, the advancements being made in quantum repeater technology signal a significant leap forward, bringing this transformative technology closer to a tangible reality.
Read the Full Popular Mechanics Article at:
[ https://www.popularmechanics.com/science/a69514722/quantum-teleporation-repeater/ ]













