The Science Behind the Northern Lights: Why Their Colors Matter
- 🞛 This publication is a summary or evaluation of another publication
- 🞛 This publication contains editorial commentary or bias from the source



The Science Behind the Northern Lights: Why Their Colors Matter
Every winter, on a clear night in the high‑latitudes of Canada, Scandinavia, or Alaska, a ribbon of light snakes across the sky. The aurora, affectionately known as the Northern Lights, has fascinated humanity for millennia. Yet, what many people see as a mystical glow is the result of a very tangible chain of events that begins in the Sun’s atmosphere and ends in Earth’s own air.
1. A Quick Primer on Auroras
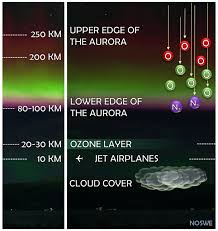
The aurora is produced when charged particles from the solar wind—protons, electrons, and alpha particles—interact with Earth’s magnetic field and are funneled toward the magnetic poles. When these energetic particles collide with atoms and molecules in the upper atmosphere (the thermosphere, roughly 80–600 km above the surface), they knock electrons into excited states. As those electrons fall back to lower energy levels, they emit photons of specific wavelengths, creating the dazzling colors we see.
Scientists study auroras using satellites, ground‑based all‑sky cameras, and rocket probes. The NOAA Space Weather Prediction Center (https://www.swpc.noaa.gov/) and NASA’s aurora website (https://www.nasa.gov/aurora) provide real‑time data and educational resources that help researchers and the public understand the space‑weather conditions that give rise to these luminous displays.
2. What Determines the Color?
The color of an aurora is dictated by:
| Color | Primary Emission Line(s) | Altitude | Primary Atmospheric Species | What It Tells Us |
|---|---|---|---|---|
| Green | 557.7 nm (O I) | 100–300 km | Neutral oxygen | Most common; indicates moderate energy particles |
| Red | 630.0 nm (O I) | 300–600 km | Neutral oxygen | Higher altitude; often appears after green subsides |
| Blue/Violet | 391.4 nm, 427.8 nm (N₂⁺) | 80–200 km | Ionized nitrogen | Requires high‑energy electrons; rare |
| White | Combination of many lines | 80–600 km | Nitrogen & oxygen | Seen when particle energy is very high |
Green is by far the most familiar auroral color. It arises when neutral oxygen atoms at about 557.7 nm re‑emit energy after being excited by solar wind electrons. The green glow typically peaks between 120–200 km altitude, giving aurora watchers a reliable indicator of where the interaction is most intense.
When the auroral activity moves higher, oxygen at 630.0 nm produces a faint red hue. The red emission requires longer energy absorption times and thus is only strong at altitudes where the atmosphere is less dense. Consequently, red auroras are most commonly seen after an evening of intense green light, as the magnetosphere gradually relaxes.
The rare blue or violet auroras are produced by ionized nitrogen (N₂⁺) and appear at lower altitudes. Their appearance signals the presence of extremely high‑energy electrons that can penetrate deeper into the atmosphere. Because of their rarity, blue auroras are often associated with the most dramatic substorms.
A white aurora—though technically a blend of green, blue, and red—indicates the presence of a wide range of particle energies and can signal a powerful space‑weather event. The mix of wavelengths creates a luminous cloud that sometimes looks like a shimmering cloud of rainbows.
3. Linking Color to Space Weather
Auroral colors are not just aesthetic—they are diagnostic. By measuring the relative intensities of green and red emissions, scientists can infer the energy spectrum of incoming electrons. A higher red/green ratio often correlates with a larger, more prolonged substorm. This is why forecasters use the Kp index (ranging from 0 to 9) and auroral oval maps (available from NOAA’s Space Weather website) to predict when a spectacular red aurora might take the sky.
The NASA Space Physics Data Facility hosts a wealth of data that scientists use to model these processes. One recent study, published in Geophysical Research Letters, examined the correlation between the green‑to‑red ratio and the intensity of the solar wind’s interplanetary magnetic field (IMF). The research showed that a southward IMF component is especially conducive to producing red auroras at high latitudes.
4. Cultural and Historical Context
While the science is compelling, the aurora has a rich cultural history. Indigenous peoples of the Arctic long used auroral observations to forecast weather changes and hunting seasons. European explorers, such as the Vikings, documented the lights in runic inscriptions, hinting at their significance before the age of space physics.
The name “aurora” comes from the Roman goddess of dawn, a fitting metaphor for a phenomenon that paints the sky at twilight. Whether you’re a seasoned space‑weather analyst or a casual stargazer, understanding the science behind the colors adds a layer of wonder to this age‑old spectacle.
5. Where to Learn More
- NASA Auroras: https://www.nasa.gov/aurora – Offers interactive animations and data on auroral physics.
- NOAA Space Weather Prediction Center: https://www.swpc.noaa.gov/ – Real‑time alerts and forecasts for auroral activity.
- Auroral Spectroscopy Studies: Many papers are available through the NASA ADS (https://ui.adsabs.harvard.edu/), such as “Spectral Signatures of Auroral Emissions in the 557‑630 nm Range.”
Next time you’re outside at a high latitude, keep an eye on the color palette of the sky. The green swirls, the faint red glow, or, if you’re lucky, a splash of blue will tell you a story about solar particles traveling millions of kilometers, the dance of Earth’s magnetic field, and the atoms in our own atmosphere dancing to the beat of the cosmos.
Read the Full WOWT.com Article at:
[ https://www.wowt.com/2025/11/12/science-behind-northern-lights-meaning-different-colors/ ]
